FAQ
Q: What was the main goal of this study?
A: We conducted a head-to-head comparison of commercially available rapid serology tests (also known as lateral flow assays - LFAs - or immunochromatographic strip tests - ICTs) and ELISA antibody tests. We included an evaluation of test performance by time from symptom onset, disease severity and other clinical features. Our goal is to highlight reliable assays to enable broader testing.
Q: What is the “best” COVID-19 rapid serology antibody test, based off of this study?
A: The goal of our study was not to recommend an ‘absolute best’ or ranked list of rapid serology antibody tests, but rather to provide well-controlled performance data to highlight a number of high-quality assays to inform the medical community and government organizations.
Q: Will you include data of additional models of commercially available rapid serology tests, in your future work?
A: Yes, new data comparing additional rapid serology tests will be regularly posted on this website, as new data becomes available.
Q: What are the main types of tests used for COVID-19?
A: This study uses three main types of COVID-19 tests:
- Rapid serology antibody tests (ICT, LFA)
- Enzyme-linked immunosorbent assays (ELISA)
- Real-time polymerase chain reaction (RT-PCR) swab tests
Rapid serology tests and ELISAs detect the presence of IgG/IgM antibodies produced by the immune system in response to a SARS2-CoV-2 infection, whereas RT-PCR swab tests are molecular tests that detect the presence of SARS2-CoV-2 viral RNA. A more in-depth comparison of these three main types of COVID-19 tests is shown below:
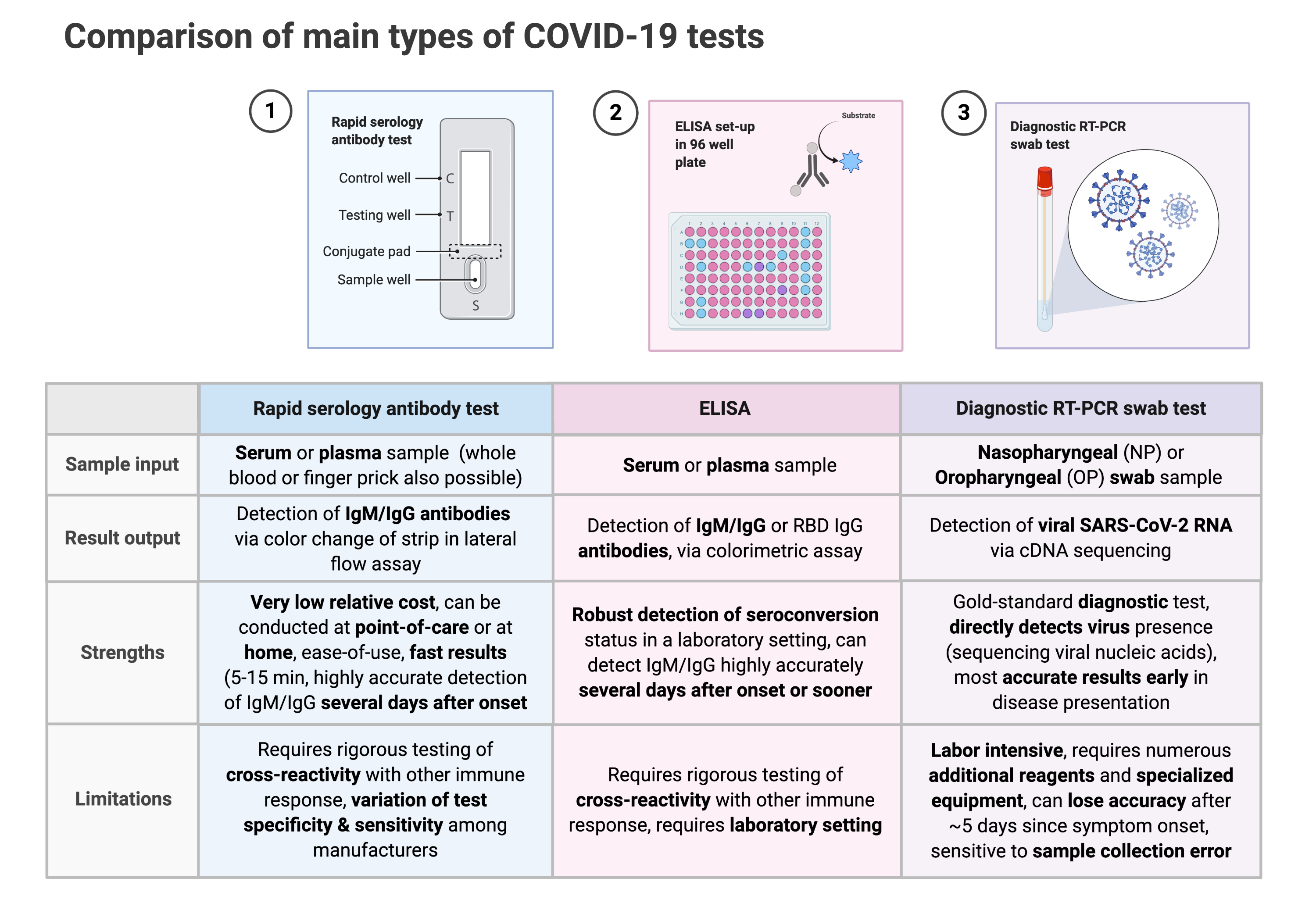
In particular, we compared the performance (sensitivity, specificity) of rapid serology tests and ELISAs by time from symptom onset, disease severity and other clinical features.
Q: Why is it important to expand COVID-19 rapid serology antibody testing? What can these types of tests tell us that the more commonly used RT-PCR swab tests cannot?
A: Recently, the US FDA has written about the importance of expanding COVID-19 rapid serology antibody testing (in a statement by FDA Commissioner Stephen M. Hahn, M.D).
Current diagnostic testing for COVID-19 has relied on labor-intensive molecular techniques (RT-PCR swab tests) and has generally been reserved for patients whose illness severity, age and/or comorbidities place them at high risk of severe disease. Antibody testing for SARS-CoV-2, including IgM and IgG responses, will enable us to understand the prevalence and incidence of SARS-CoV-2 infections.
- To provide complementary low-cost, low-labor COVID-19 test data, to allow for health care personnel and other essential workers with potential protective immunity to return to work and daily life more safely and quickly (Weitz, Beckett et al. 2020).
- To help identify individuals with robust antibody responses, whose serum or plasma may potentially serve as a therapeutic option for severe COVID-19 disease - although this has not yet been confirmed (Cheng, Wong et al. 2005, Duan, Liu et al. 2020, Roback and Guarner 2020, Shen, Wang et al. 2020).
Recent work has also described that SARS-CoV-2 viral shedding often occurs in early presymptomatic stages, which could affect the dynamics and accuracies of diagnostic SARS-CoV-2 RT-PCR swab tests (He et al. 2020)—especially if patients do not seek molecular diagnostic testing until several days after symptom onset. COVID-19 rapid serology antibody tests instead detect IgM/IgG antibodies, which are generated 1-3 weeks after symptom onset.
Combined, a SARS-CoV-2 RT-PCR swab test coupled with a COVID-19 rapid serology antibody test would provide a more complete picture of the progression of the COVID-19 disease, in a given individual and across populations.
Q: Where are these COVID-19 rapid serology antibody tests manufactured? Are they endorsed by the US FDA?
A: COVID-19 rapid serology antibody tests are being manufactured and distributed in numerous countries worldwide (non-exhaustive list here). A full list of FDA EUA rapid serology antibody tests (and other molecular diagnostic tests and ELISAs) is listed here.
Q: How do COVID-19 rapid serology antibody tests work?
A: Rapid serology antibody tests work by screening a sample of blood, serum, or plasma for the presence of SARS-CoV-2-specific antibodies. The rapid test uses the principle of capillary action to pull the sample across the strip into the test’s detection zone. As the sample progresses across the detection zone, antibodies in the sample may bind to recombinant SARS-CoV-2 antigens immobilized on the test membrane. Depending on the content of the sample, the test will display one or more colored lines, indicating binding and thus presence of antibodies generated in response to a SARS-CoV-2 infection. The general workflow of COVID-19 rapid serology antibody tests is illustrated below:
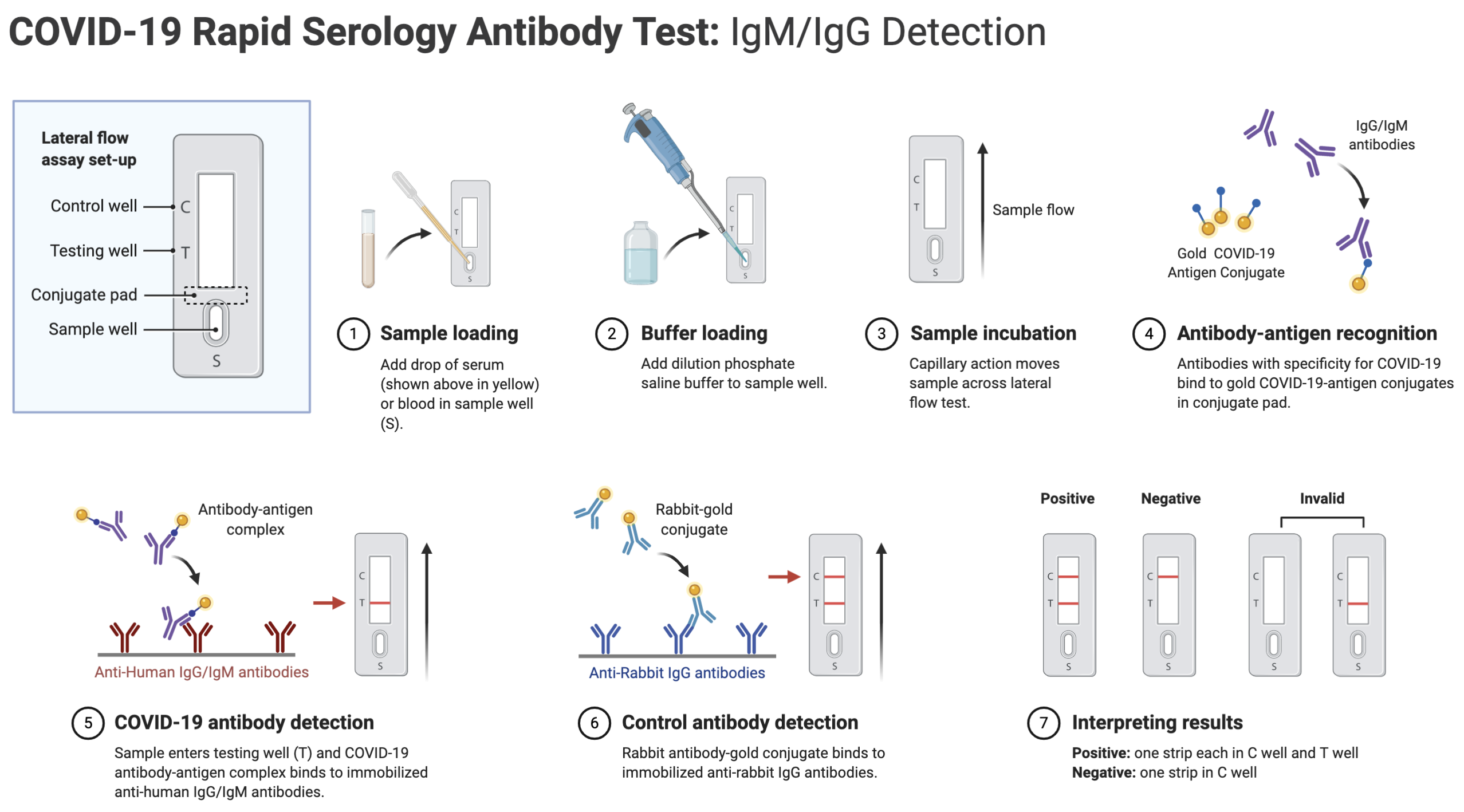
Q: How do COVID-19 rapid antibody se,rology tests compare to RT-PCR swab tests?
A: RT-PCR swab tests screen for the presence of SARS-CoV-2 at the swab site — a positive result indicates an active infection. By contrast, rapid serology tests are able to determine if you have previously been infected with SARS-COV-2 and have mounted an antibody response against the virus. Therefore, a positive rapid serology test means that you may have been infected with SARS-COV-2 in the past, but does not necessarily indicate active infection. This is different from the standard RT-PCR swab tests, which can detect active infections but not past infections. A positive RT-PCR result indicates that you may be actively infected with SARS-COV-2. The general workflow of COVID-19 diagnostic RT-PCR swab tests is illustrated below:
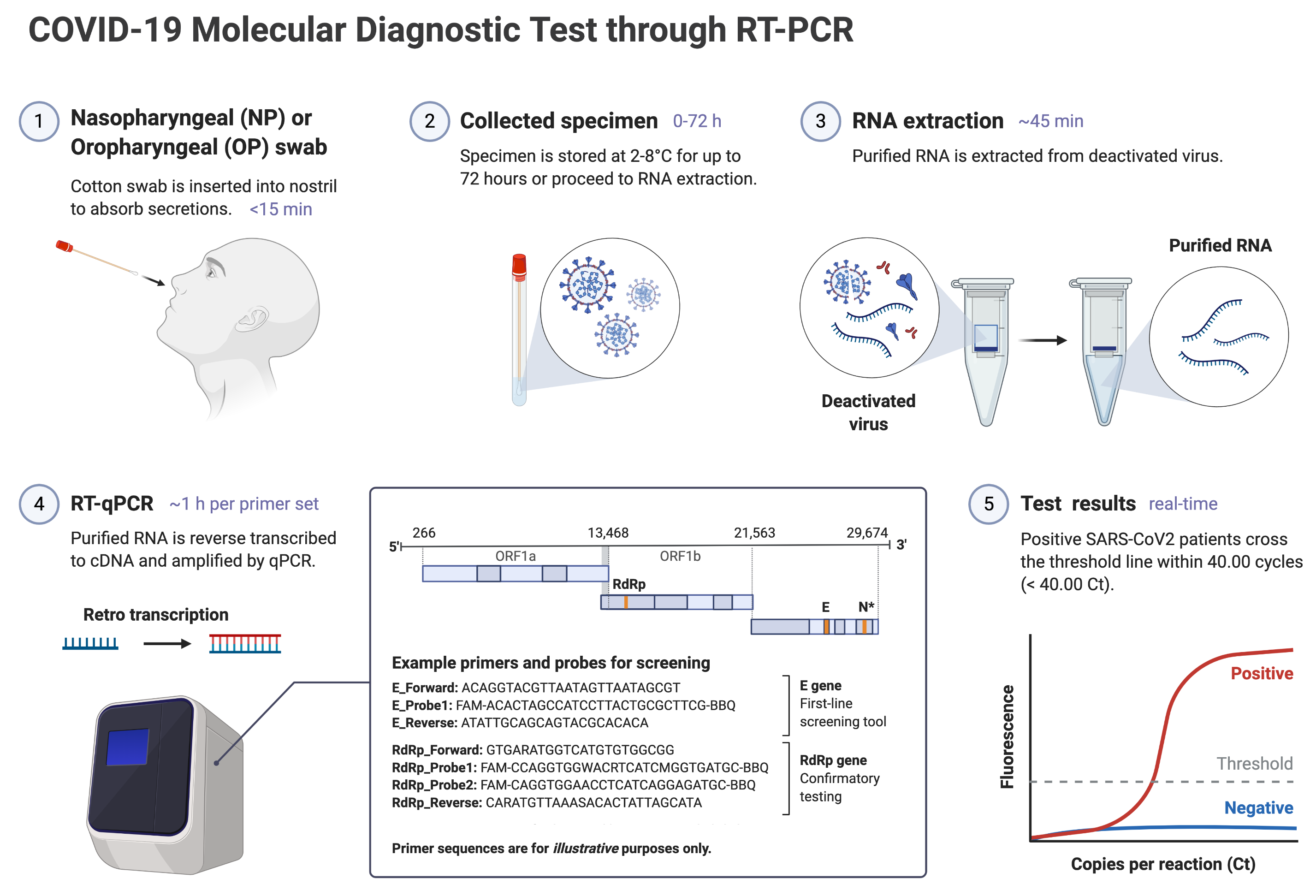
Q: Roughly how much do COVID-19 rapid antibody tests cost? Compared to other leading types of tests?
A: COVID-19 rapid antibody tests have an average cost of $10/test, and the immunochromatographic strip rapid serology tests (ICTs) require no additional equipment beyond a lancet, alcohol pad, and running buffer. In contrast, ELISAs and RT-PCR swab tests require additional laboratory overhead and equipment.
Figures made with Biorender.com